Real QULIPTA patient.

QULIPTA® Mechanism of Action
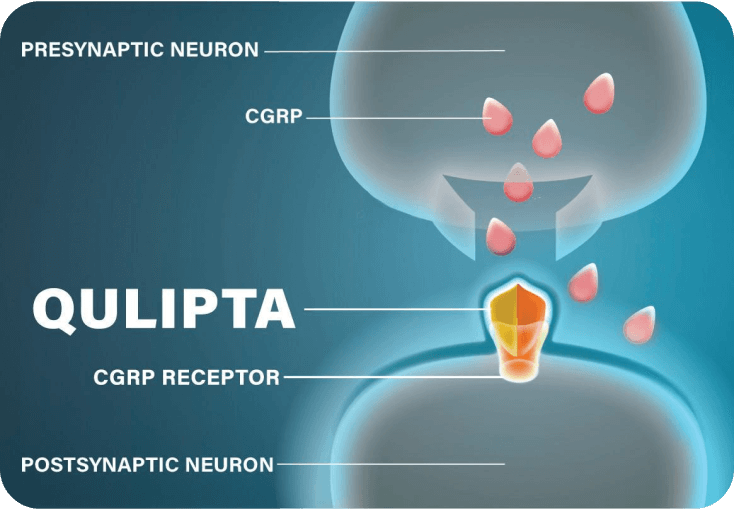
QULIPTA MECHANISM OF ACTION
QULIPTA—specifically designed to help prevent migraine attacks
CGRP levels increase during migraine attacks
While research into the exact mechanism that causes migraine is ongoing, CGRP is known to play a role. When CGRP binds to CGRP receptors, it causes pain, inflammation, and vasodilation.6-8
QULIPTA is the only once-daily oral preventive CGRP receptor antagonist
QULIPTA was specifically designed to help prevent migraine attacks. It works by directly targeting the CGRP receptor and blocking CGRP from binding.1
In a 2024 survey of migraine patients taking preventive treatment (N=425)2*
of patients said they prefer a medication that is specifically designed to treat only migraine2†
*Source: Online survey conducted by Ipsos Healthcare on behalf of AbbVie Inc., between June 16, 2024 and July 16, 2024, among 425 adults aged 18-64, who were current users of QULIPTA, CGRP mAbs, traditional oral preventive medications, or Nurtec® ODT for preventive use at the time of the survey.
†Survey respondents could choose from the following to indicate their agreement with the provided statement: strongly disagree, disagree, somewhat disagree, neither disagree nor agree, somewhat agree, agree, or strongly agree. The percentage represents those who answered strongly agree, agree, and somewhat agree.
CGRP=calcitonin gene-related peptide; mAb=monoclonal antibody.
