Real QULIPTA® patient.
EPISODIC MIGRAINE
Continuous
Control
Continuous control with migraine day reductions across 12 weeks1
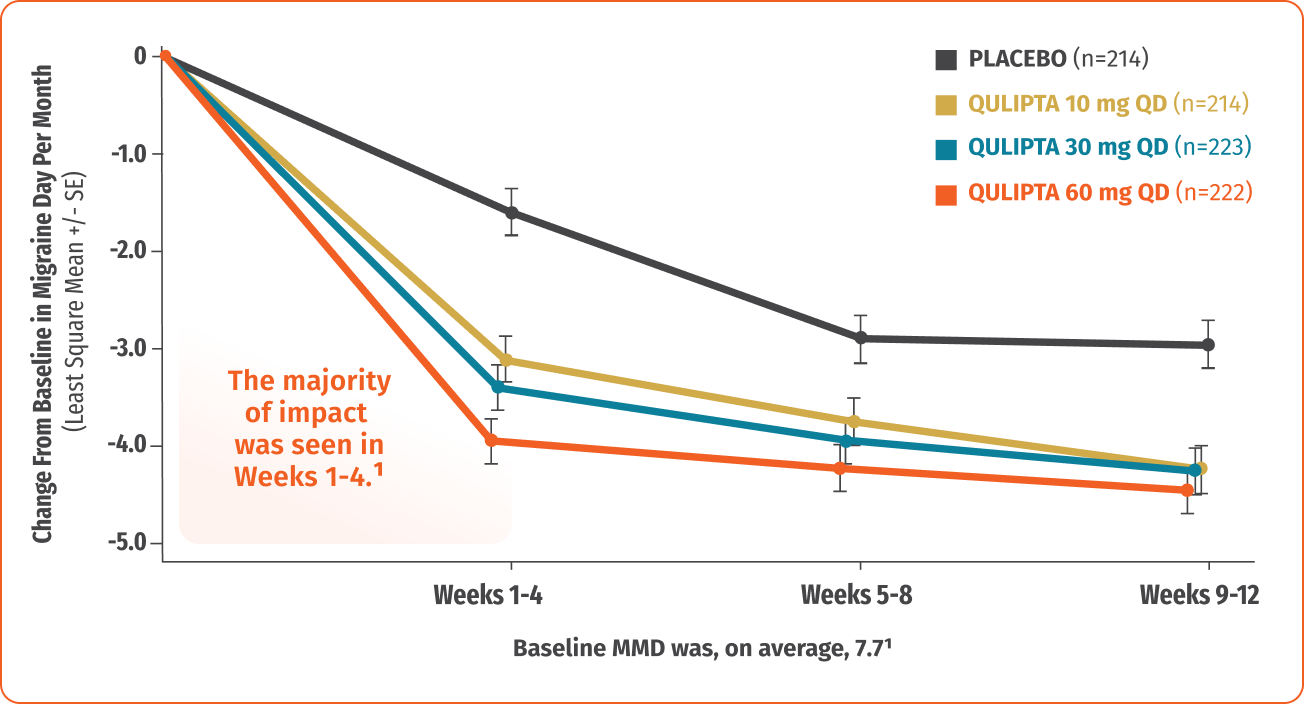
MMD=Monthly Migraine Days.
Open-label, long-term safety study for EM
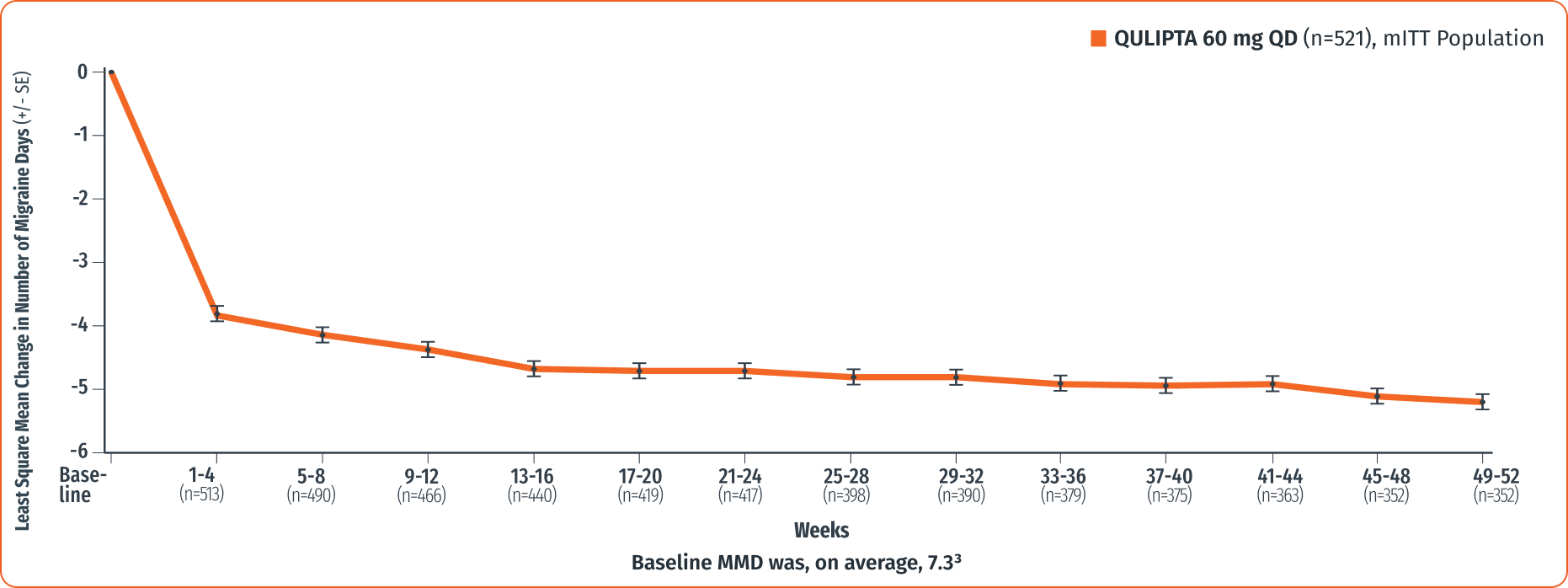
Reductions observed across 52 weeks
Exploratory endpoint: Change from baseline in MMD across 52 weeks2,3
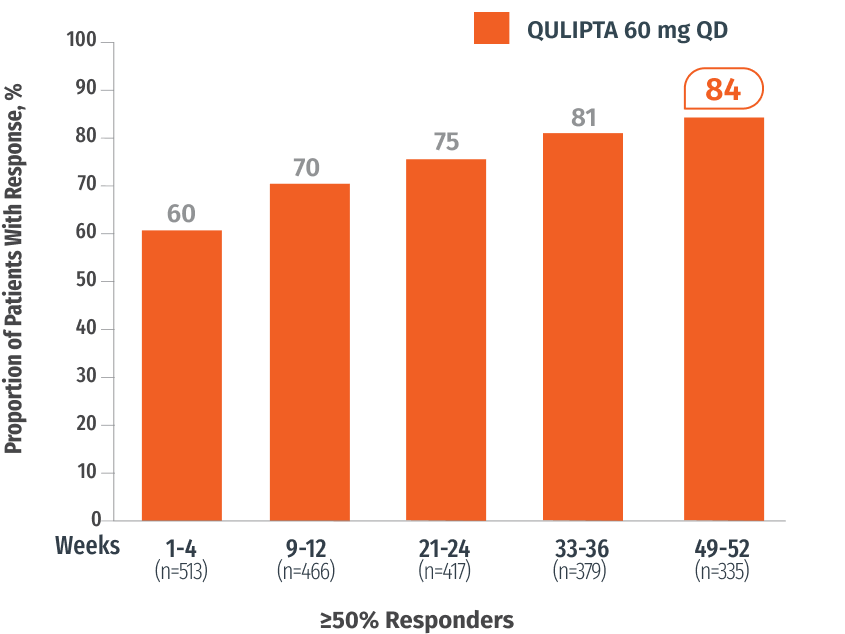
Exploratory endpoint: Percentage of patients who achieved ≥50% MMD reduction at specified intervals3
In Weeks 49-52
84%
of patients achieved ≥50%
MMD reduction3
(n=335)
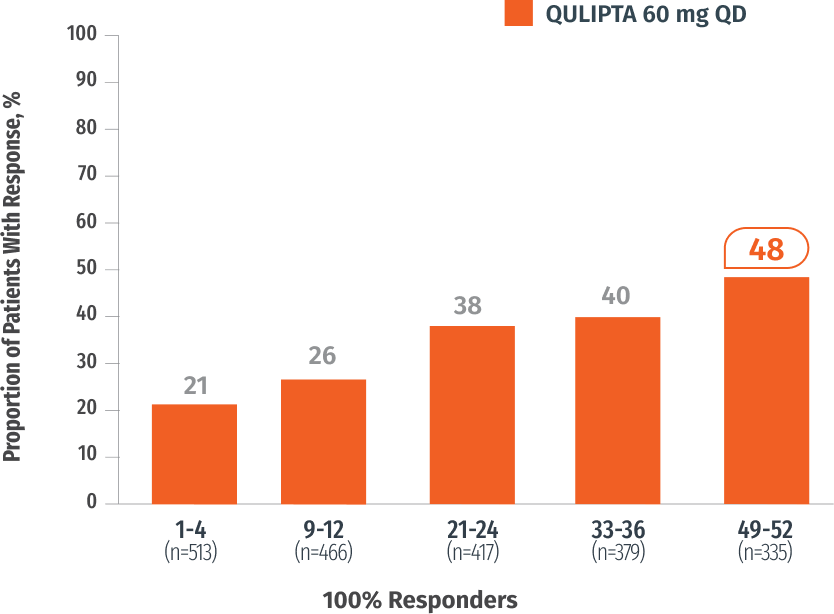
Exploratory endpoint: Percentage of patients who achieved 100% MMD reduction at specified intervals3
In Weeks 49-52
48%
of patients achieved 100%
MMD reduction3
(n=335)
LIMITATIONS: These are observations from the 52-week, open-label safety study for which efficacy measures were not an endpoint. 31/543 (5.7%) discontinued due to adverse events.3 This open-label safety study was not blinded, not controlled, and included inherent self-selection bias for remaining in the trial. Results should be interpreted with these factors in mind.
EM=episodic migraine; mITT=modified intention-to-treat; MMD=Monthly Migraine Days.

