EPISODIC MIGRAINE Continuous Control
12 WEEKS
Continuous control with migraine day reductions across 12 weeks1

EM=episodic migraine; MMD=Monthly Migraine Days.
ADDITIONAL ENDPOINT: DAY 1
Reductions observed as early as Day 1
Additional endpoint:
Day 1
88% of EM patients (185/211) did not have a migraine day on Day 1 after the initial dose vs 75% placebo3 (151/202)*
LIMITATIONS: The additional endpoints were pre-specified, non-ranked endpoints and were not adjusted for multiplicity. Therefore, treatment differences cannot be regarded as statistically significant.
*Proportion of participants with a migraine day on Day 1 after the first dose: 12.3% (26/211) of QULIPTA 60 mg patients had a migraine day vs 25.2% (51/202) of placebo patients.3
EM=episodic migraine.
ADDITIONAL ENDPOINT: WEEK 1
QULIPTA—reductions observed as early as Week 1
Additional endpoint: Week 1

*Change from baseline in weekly migraine days (WMD): Week 1 QULIPTA 60 mg: -1.03 WMD from 1.93 baseline; Week 1 placebo: -0.29 WMD from 1.88 baseline.
EM=episodic migraine.
LIMITATIONS: The additional endpoints were pre-specified, non-ranked endpoints and were not adjusted for multiplicity. Therefore, treatment differences cannot be regarded as statistically significant.
EXPLORATORY ENDPOINTS: 52 WEEKS
Open-label, long-term safety study for EM
Reductions observed across 52 weeks
Exploratory endpoint: Change from baseline in MMD across 52 weeks3

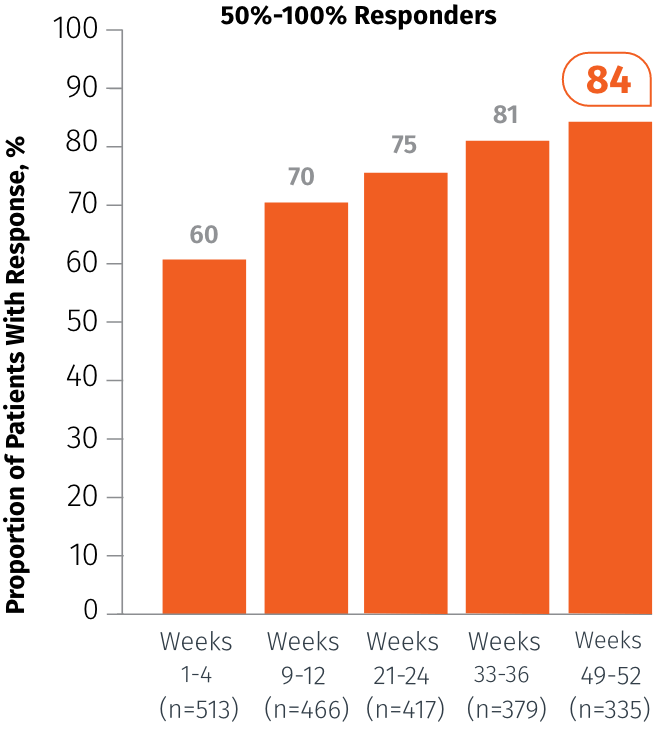
Exploratory endpoint: Percentage of patients who achieved 50%-100% MMD reduction at specified intervals3
In Weeks 49-52
84%
of patients achieved
50%-100% MMD reduction3
(n=335)
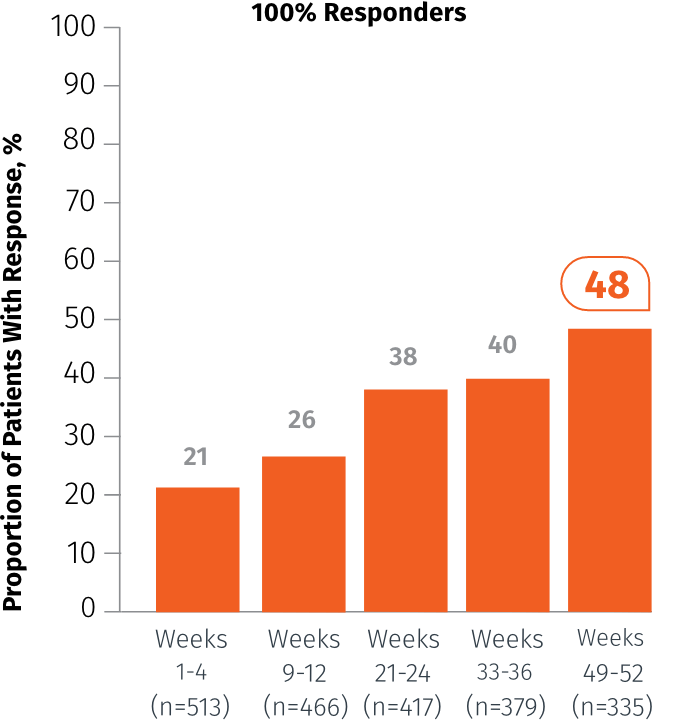
Exploratory endpoint: Percentage of patients who achieved 100% MMD reduction at specified intervals3
In Weeks 49-52
48%
of patients achieved
100% MMD reduction3
(n=335)
LIMITATIONS: These are observations from the 52-week, open-label safety study for which efficacy measures were not an endpoint. 31/543 EM patients (5.7%) discontinued due to adverse events.3 Data from this open-label safety study have limitations as the study was not blinded, not controlled, and included inherent self-selection bias for remaining in the trial. Results should be interpreted with these factors in mind.
EM=episodic migraine; mITT=modified intention-to-treat; MMD=Monthly Migraine Days.
See the safety profile of QULIPTA.
